Application & Background
Despite being banned in the United States in 1976, chlordecone continued to be used extensively as a pesticide in banana farming operations in the French West Indies, Guadeloupe and Martinique until 1993 (1). Previous studies have demonstrated that high levels of chlordecone in blood have been associated with central nervous system toxicity, liver toxicity, and adverse reproductive effects in both women and men. Other research has demonstrated that chlordecone is also a carcinogenic compound and an endocrine disrupting chemical. However, as an organochlorine compound, chlordecone is extremely resistant to environmental degradation, and significant levels of chlordecone continue to be detected in water, food, and soil in the French West Indies and French Caribbean islands (1).
As these populations continue to be exposed to chlordecone at levels associated with adverse health effects, it is of critical importance to better understand the mechanisms of toxicity of chlordecone (1). Yet, little is known about the inter-tissue distribution of chlordecone and how in situ accumulation within substructures of target organs could be contributing to observed symptoms of chlordecone toxicity (2). Previously, the Protim Core Facility and the Irset Institute at Rennes developed a quantitative matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging (MSI) method to study the inter-tissue distribution of chlordecone hydrate, which is the compound formed when chlordecone is in the presence of water (Figure 1) (2). However, this original method was found to be suitable only for quantifying chlordecone hydrate in relatively homogenous tissues, such as the rodent liver and prostate, at a relatively low spatial resolution of 80 μm (Figures 2 & 3). Attempts to image chlordecone hydrate at higher spatial resolutions yielded inconclusive results with regard to coordinating small histological structures with the biochemical signal of chlordecone hydrate. This was specifically seen in the testis, which is a highly structured tissue, with regions of the seminiferous tubules coordinated with distinct phases of spermatogenesis and sperm maturation. The testis is also known to be a target organ for chlordecone toxicity (1,3).
Figure 1. General schematic of chlordecone hydrate detection by MALDI MSI and a representative spectrum of the typical isotopic pattern.
Previous attempts to image the distribution of chlordecone hydrate in the rat testis could not distinguish the testis substructures to a sufficient degree to draw conclusions about the toxicological mechanism of chlordecone’s effects on the different regions of the seminiferous tubules (Figure 4). In order to better understand the association between chlordecone exposure and oligospermia, a novel sample preparation protocol was developed using the HTX M5 Sprayer for MSI of chlordecone hydrate in the rat testis at a higher spatial resolution to allow for the visualization of chlordecone hydrate accumulation in substructures of the testis
Figure 2. (A) Microscopic image of a liver from a mouse that was exposed to chlordecone at 8 mg/kg for 10 days then CCl4 at 0.1 mg/kg. prior to MALDI imaging, with areas of health and necrotic tissue annotated. (B) MALDI-TOF-TOF MS images of m/z 506.68, chlordecone hydrate, in a mouse liver in negative ion mode with DCTB matrix. Pixel size 80 µm. Scale bars are 500 µm.*
Figure 3. (A) Images of H&E stained prostrates from rats that were exposed to chlordecone at 5 mg/kg/week for 1, 15 or 20 weeks with the tumor and stroma annotated. (B) MALDI FT-ICR MS images of the prostates in negative ion mode with DCTB matrix. Pixel size 80 µm. (C) Merged images of the H&E stained prostate and the MALDI FT-ICR MS images. It is clear that chlordecone hydrate accumulated in the tumor regions of the mouse prostate. Scale bars are 1 mm.
Experimental
experimental design
Adult male Sprague-Dawley rats and C57Bl/6 mice were purchased from Janvier Labs (Le Genest-Saint-Isle, France). Mice were exposed to chlordecone at 8 mg/kg of body weight by daily gavage with olive oil containing chlordecone for 10 days. Mice were then subjected to a gavage with CCl4 at 0.1 mg/kg of body weight to induce liver necrosis. Rats were exposed to chlordecone at 5 mg/kg of body weight by weekly gavage with olive oil containing chlordecone for 1, 15 or 20 weeks. Mice and rats were sacrificed 72 h after the last gavage with chlordecone. At necropsy, organs were removed, frozen in liquid nitrogen and stored at -80⁰C until needed. Liver, prostate and testes tissues were sectioned at 12 μm on a Leica CM 1900 UV cryostat (Leica Microsystems GmbH, Wetzlar, Germany) and mounted on indium-tin-oxide- (ITO) coated slides (Bruker Daltonik GmbH, Bremen, Germany).
Sample Preparation
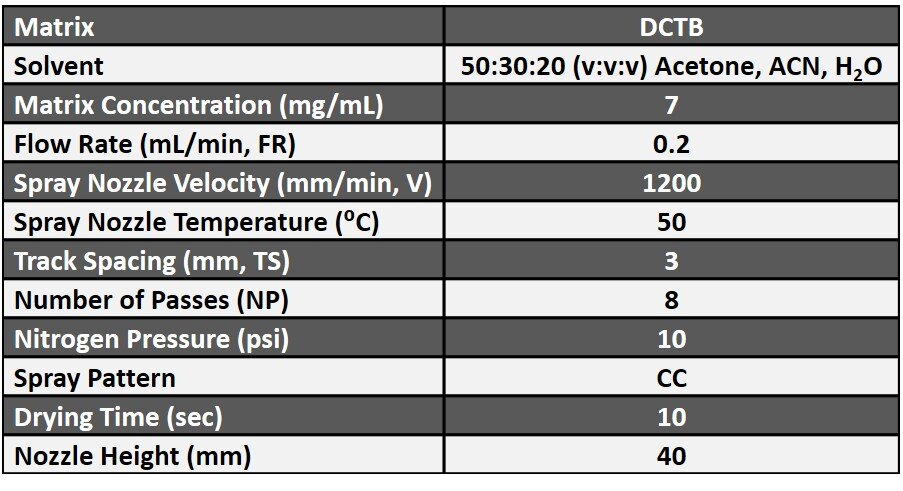
After sectioning, slides were dried in a desiccator for one hour. Slides were then sprayed with Trans-2-[3-(4 tert-butylphenyl)-2-methyl-2 propenylidene] malonitrile (DCTB) using the HTX M5 Sprayer. Unique features of the M5 Sprayer were optimized to create a drier and finer spray than the previous spraying apparatus used. These included nozzle temperature, spraying pattern, flow rate, and solvent composition. The final optimized spraying parameters are detailed in Table 1.
Table 1. Spraying parameters for DCTB matrix on rat prostate and testis tissues for the HTX M5 Sprayer.
data collection and analysis
Experiments on rat tissues were performed on a MALDI-Fourier transform ion cyclotron resonance (FT-ICR) mass spectrometer equipped with Smartbeam II 2KHz Nd:YAG laser (Bruker Daltonik, GmbH, Bremen, Germany). Experiments on mouse liver were performed on a MALDI-TOF-TOF mass spectrometer equipped with Smartbeam II 2KHz Nd:YAG laser (Bruker Daltonik, GmbH, Bremen, Germany). Data were collected in negative ion mode at 80 or 50 µm spatial resolution. Raw data files were exported in imZML format and imported into SCiLs software for image processing (Bruker Daltonik, GmbH, Bremen, Germany).
results
Improvements in Crystal Size and Homogeneity using the
Figure 4. MALDI FT-ICR MS images of chlordecone hydrate m/z 506.68 ± 10 Da in testis tissue from a rat that was exposed to chlordecone at 5 mg/kg/week for 15 weeks in negative ion mode with DCTB matrix prepared with the old sprayer device. Ion image is overlaid on a microscopic image. Pixel size 50 µm. Scale bar is 4 mm.
HTX M5 Sprayer
The heated nozzle of the M5 Sprayer allowed for a higher flow rate and higher percentage of organic solvent to be used compared to the spraying conditions originally used to image chlordecone hydrate in the rat testis in Figure 4 to create smaller DCTB crystals. In addition, the ability to program a criss-cross spray pattern and drying time also resulted in a more homogenous crystal layer compared with the spraying apparatus previously used (Figure 5).
Figure 5. (A) DCBT crystals on rat testis tissue prepared with the older spraying device. (B) DCBT crystals on rat testis tissue prepared with the HTX M5 Sprayer.
Imaging Chlordecone in Testis Substructures at 50 µm Spatial Resolution
In Figure 4, it can be seen that chlordecone hydrate detection and localization at 50 µm spatial resolution is not really possible on the testis tissue with the original sample preparation conditions. There is no clear distinction in the MALDI image between the lumen of tubules and interstitial tissue or between the upper section and the base of tubule and the interstitial tissue. However, using the optimized M5 Sprayer sample preparation protocol, as seen in Figure 6, chlordecone hydrate can be detected at 50 µm spatial resolution on the rat testis tissue. It is clear that chlordecone is most concentrated in the interstitial tissue of the testis, and least concentrated in the lumen of the seminiferous tubules.
Figure 6. (A) MALDI FT-ICR MS images of chlordecone hydrate m/z 506.68 ± 10 Da in rat testis tissue in negative ion mode from a rat that were exposed to chlordecone at 5 mg/kg/week for 15 weeks in negative ion mode with DCTB matrix prepared with the HTX M5 Sprayer. Ion image is overlaid on a microscopic image. (B) Inset image at increased magnification in order to demonstrate the detection of chlordecone hydrate is distinctly visible in various substructures of testis. Pixel size 50 m.µm. Scale bar is 3 mm.
Conclusions
Paracelsus famously said, “The dose makes the poison.” However, as our understanding of toxicity has grown, we also know that the site of accumulation of a pollutant is also a crucial datum in relating environmental exposures to adverse health outcomes. This novel sample preparation protocol for MALDI MSI of chlordecone in rat testis tissue represents a workflow that can provide both quantitative and intertissue-specific location of a dangerous chemical to which many people in the French West Indies continue to be exposed.
The tissue images and MS data presented here were provided by Dr. Mélanie Lagarrigue, Dr. Régis Lavigne, and Dr. Charles Pineau at the Université de Rennes 1, F-35043 Rennes, France.
References
(1) Multigner L, Kadhel P, Rouget F, Blanchet P, Cordier S. Chlordecone exposure and adverse effects in French West Indies populations. Environ Sci Pollut Res Int. 2016 Jan;23(1):3-8. doi: 10.1007/s11356-015-4621-5.
(2) Lagarrigue M, Lavigne R, Tabet E, Genet V, Thomé JP, Rondel K, Guével B, Multigner L, Samson M, Pineau C. Localization and in situ absolute quantification of chlordecone in the mouse liver by MALDI imaging. Anal Chem. 2014 Jun 17;86(12):5775-83. doi: 10.1021/ac500313s.
(3) Lagarrigue M, Becker M, Lavigne R, Deininger SO, Walch A, Aubry F, Suckau D, Pineau C. Revisiting rat spermatogenesis with MALDI imaging at 20-micron resolution. Mol Cell Proteomics. 2011 Mar;10(3):M110.005991. doi: 10.1074/mcp.M110.005991.